French ministry of health-sponsored randomized control trial1
Objective
This independent study conducted by the French Ministry of Health examined efficacy of Enterra Therapy in the treatment of chronic, intractable nausea and vomiting, with or without gastroparesis.
Design
Prospective, large, multi-center, double-blinded, 1:1 randomized trial with cross-over study design across 19 centers in France.
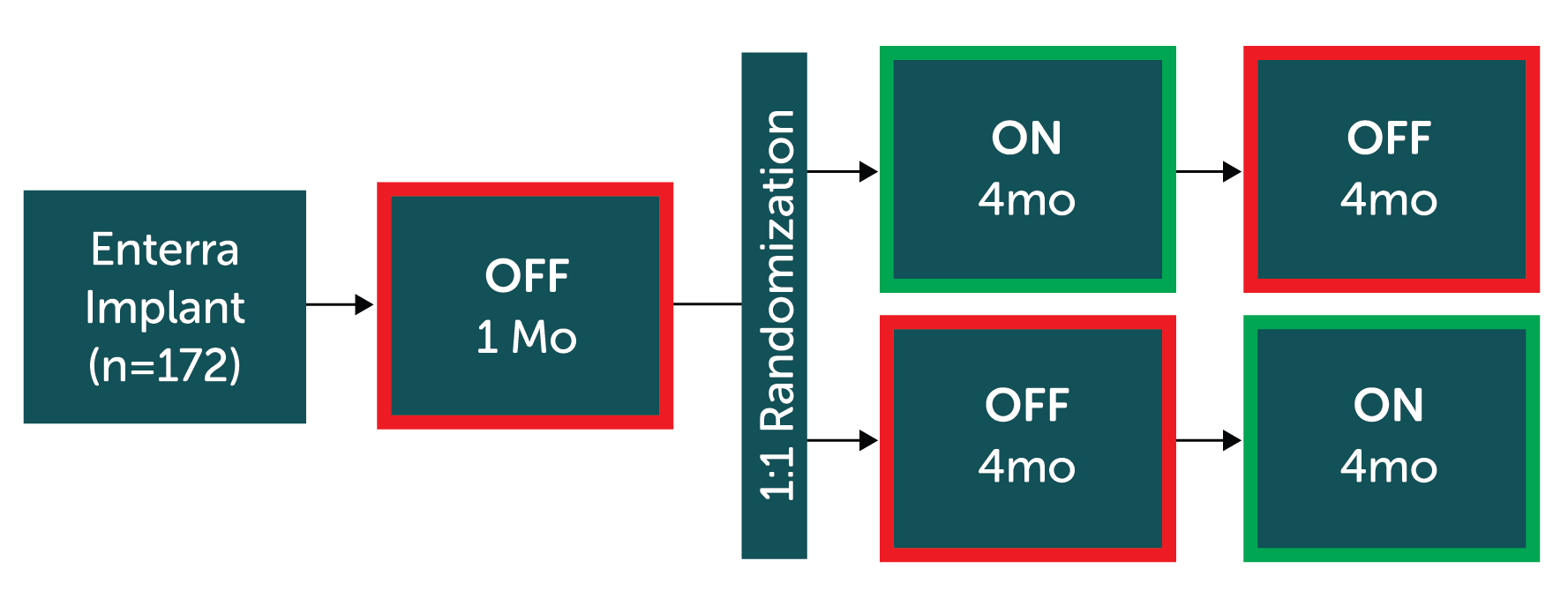
Primary endpoint
Vomiting Score (measured 0-4 numeric scale), QOL.
Results
- This study, the largest RCT conducted on Enterra Therapy to date, showed statistically significant improvement in symptoms at 9 months.
- A portion of the study subjects were off-label, and the results for those patients are not presented here.
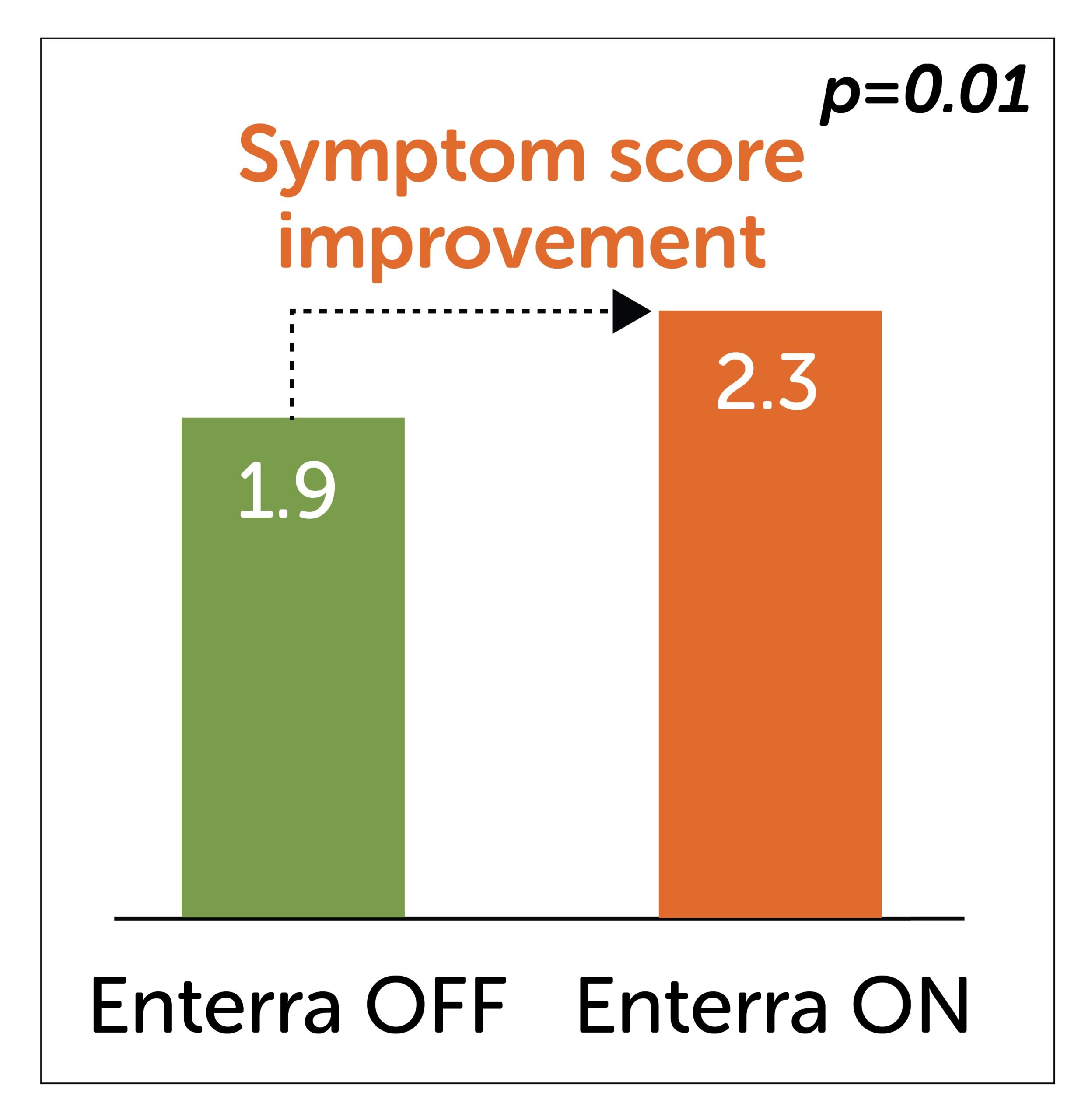
Trial-specific vomiting score
(Higher score represents improved symptoms)
| Scoring | Frequency of vomiting |
|---|---|
| 0 | Several vomiting episodes a week |
| 1 | No more than 1 vomiting episodes a week |
| 2 | At least 1 vomiting episode a month |
| 3 | Less than 1 vomiting episodes a month |
| 4 | No vomiting episode |
- Ducrotte P, Coffin B, Bonaz B, et al. Gastric Electrical Stimulation Reduces Refractory Vomiting in a Randomized Crossover Trial. Gastroenterology. 2020;158(3):506-514.e2. doi:10.1053/j.gastro.2019.10.018.
