Living with Enterra® Therapy
Taking back your seat at the table
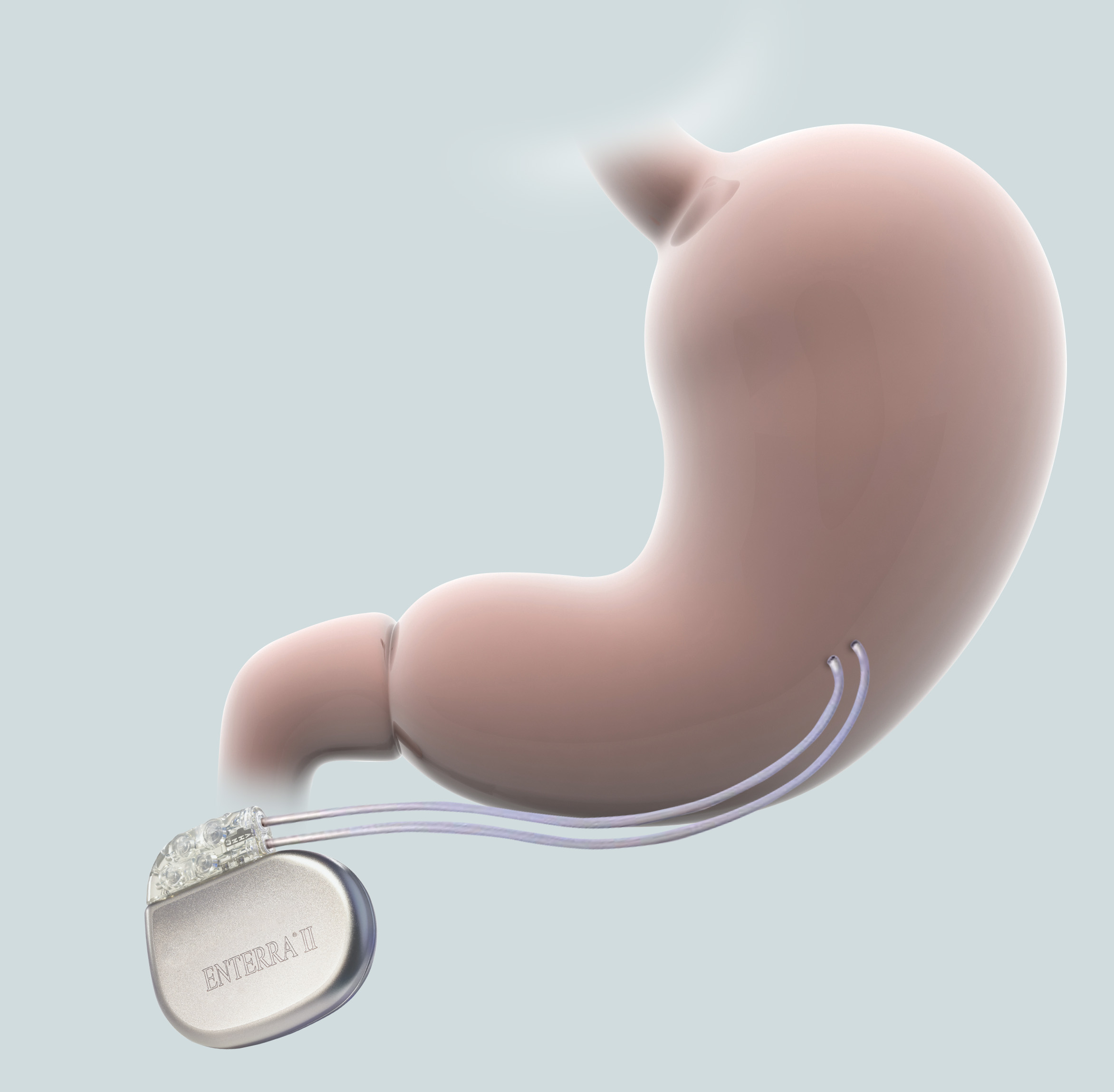
Once you’ve received your Enterra® System, your doctor will provide ongoing management of your therapy. Below, you’ll find helpful tips and information about living your daily life with Enterra Therapy.
If you’re considering Enterra Therapy, answer a few questions to see if it may be right for you.

Following your procedure
After you receive your Enterra System, it’s normal to have some pain at the incision site for up to 6 weeks. Your doctor will help you manage any pain if it becomes severe.
When recovering, be sure to follow your doctor’s advice. This will likely include avoiding heavy lifting or activities that involve excessive or repetitive bending, twisting, bouncing, or stretching, as these movements could damage or displace your leads or affect the neurostimulator’s ability to provide stimulation.
Ask your doctor when it’s safe to return to normal daily activities like work, travel, and hobbies.
Check-ups and monitoring
Once you’ve received your Enterra System, your doctor will schedule a series of in-office appointments, typically at 1 week after surgery; 1, 3, and 6 months after surgery; and as needed after that.
During these appointments, your doctor will monitor how you’re adjusting to life with Enterra Therapy and modify your neurostimulator settings to ensure you’re receiving the right level of stimulation from your system.
What to expect from Enterra Therapy
You should not feel any stimulation from your Enterra System. Call your doctor if symptoms return or if you have new or unusual abdominal pain, cramping, nausea, or vomiting, as these may indicate a problem with your implanted system.
It’s important to remember that while Enterra Therapy is intended to reduce the chronic, drug-resistant nausea and vomiting associated with gastroparesis*, it is not a cure. Not all patients will experience relief with Enterra Therapy, and results will vary amongst those that do.
Often, a combination of treatments is necessary to control symptoms of gastroparesis. Therefore, it’s important to follow the advice of your gastroenterologist, endocrinologist, or primary care doctor—including any specialized diet and nutrition recommendations.
Your Enterra System patient information
Before you leave the hospital, you’ll receive an Enterra System patient manual and temporary registration card. Please read the manual and carry the registration card with you until you receive your permanent card in the mail.
Always carry your device registration card with you and make a copy of the card for each of your health care providers (doctors, dentists, physical therapists, nurse practitioners, etc.). Contact Enterra Medical if your address or physician changes.
Additionally, tell your doctor if your address changes. If you change doctors, be sure to have your medical history sent to your new doctor.
Electrical or magnetic equipment
Most electrical devices and magnets will not affect the operation of your Enterra System.
However, some equipment found in homes, workplaces, public places, and medical and dental facilities may damage your neurostimulator, interfere with its operation, or harm you. It’s important to talk with your doctor before having a medical procedure, dental procedure, or diagnostic test.
Patients with an Enterra System cannot have diathermy of any kind.

Patients with an implanted Enterra II System may have an MRI scan of some body parts under certain conditions. Consult your doctor to determine if you are eligible for MRI examination or refer to your Enterra System Patient Manual.
Your Enterra System battery
The Enterra System is powered by a battery sealed inside your neurostimulator. Over time, the battery will run down and need to be replaced through another surgical procedure with your doctor.
Before the replacement surgery, ask your doctor to request prior authorization from your health insurance company to help cover the cost of the procedure.
During the surgery, your doctor will remove the neurostimulator and implant a new one. Your implanted leads will also be checked to make sure they are working properly. If they are, the new neurostimulator will be connected to the leads that are already in place. If the leads are not working as they should be, they will be replaced too.
The information provided on this site is for general educational purposes only and is not a substitute for professional medical advice, diagnosis or treatment. Always talk to your doctor about the best treatment options for your individual situation.
MKT-D-0003, Rev AC
IMPORTANT SAFETY INFORMATION
Enterra Therapy for treatment of chronic, resistant to medication nausea and vomiting associated with gastroparesis caused by diabetes or an unknown origin in patients aged 18 to 70 years: patients should always discuss potential risks and benefits of the device with their physician.
*HUMANITARIAN DEVICE
Authorized by Federal law for use in the treatment of chronic intractable (drug refractory) nausea and vomiting secondary to gastroparesis of diabetic or idiopathic etiology in patients aged 18 to 70 years. The effectiveness of this device for this use has not been demonstrated. What does this mean?
